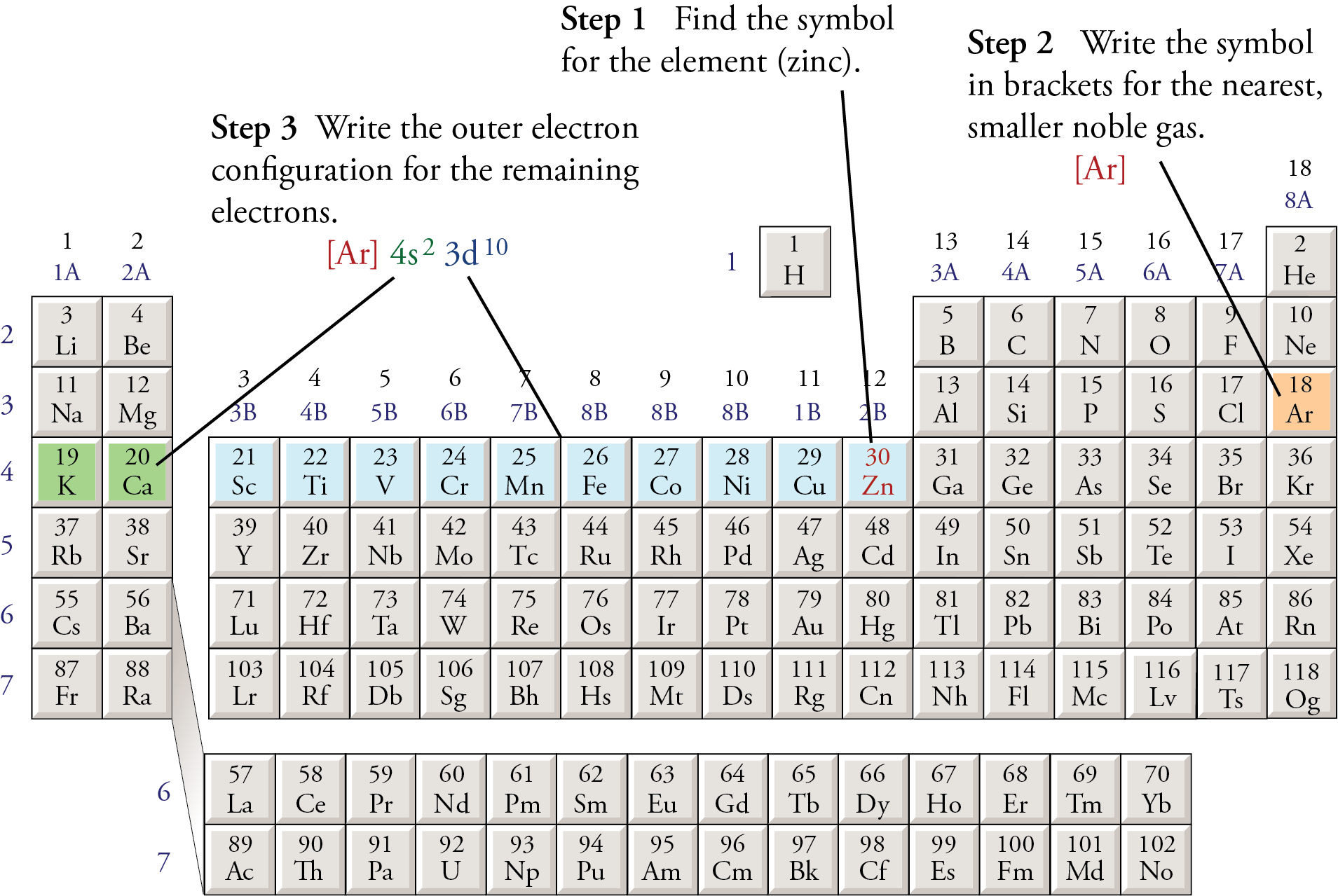
Abbreviated Electron Configurations
-
Follow these steps to write abbreviated electron configurations.
Step 1 Find the symbol for the element on a periodic table.
For example, to write an abbreviated electron configuration for zinc atoms, we first find Zn on the periodic table (see below).
Step 2 Write the symbol in brackets for the noble gas located at the far right of the preceding horizontal row on the table.
For zinc, we move up to the third period and across to Ar (see below). To describe the first 18 electrons of a zinc atom, we write
[Ar]
Step 3 Move back down a row (to the row containing the element you wish to describe) and to the far left. Following the elements in the row from left to right, write the outer-electron configuration associated with each column until you reach the element you are describing.
For zinc, we need to describe the 19th through the 30th electrons. The atomic numbers 19 and 20 are in the fourth row of the s block, so the 19th and 20th electrons for each zinc atom enter the 4s2 sublevel. The atomic numbers 21 through 30 are in the first row of the d block, so the 21st to the 30th electrons for each zinc atom fill the 3d sublevel (see below). Zinc, with atomic number 30, has the abbreviated configuration
[Ar] 4s2 3d10